Prostate cancer
| Prostate cancer | |
|---|---|
| Classification and external resources | |
 Micrograph of prostate adenocarcinoma, acinar type, the most common type of prostate cancer. Gleason pattern 4. Needle biopsy. H&E stain. |
|
| ICD-10 | C61. |
| ICD-9 | 185 |
| OMIM | 176807 |
| DiseasesDB | 10780 |
| MedlinePlus | 000380 |
| eMedicine | radio/574 |
| MeSH | D011471 |
Prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Most prostate cancers are slow growing; however, there are cases of aggressive prostate cancers.[1] The cancer cells may metastasize (spread) from the prostate to other parts of the body, particularly the bones and lymph nodes. Prostate cancer may cause pain, difficulty in urinating, problems during sexual intercourse, or erectile dysfunction. Other symptoms can potentially develop during later stages of the disease.
Rates of detection of prostate cancers vary widely across the world, with South and East Asia detecting less frequently than in Europe, and especially the United States.[2] Prostate cancer tends to develop in men over the age of fifty and although it is one of the most prevalent types of cancer in men, many never have symptoms, undergo no therapy, and eventually die of other causes. This is because cancer of the prostate is, in most cases, slow-growing, symptom-free, and since men with the condition are older they often die of causes unrelated to the prostate cancer, such as heart/circulatory disease, pneumonia, other unconnected cancers, or old age. About 2/3 of cases are slow growing "pussycats", the other third more aggressive, fast developing being known informally as "tigers".[3]
Many factors, including genetics and diet, have been implicated in the development of prostate cancer. The presence of prostate cancer may be indicated by symptoms, physical examination, prostate specific antigen (PSA), or biopsy. There is controversy about the accuracy of the PSA test and the value of screening. Suspected prostate cancer is typically confirmed by taking a biopsy of the prostate and examining it under a microscope. Further tests, such as CT scans and bone scans, may be performed to determine whether prostate cancer has spread.
Treatment options for prostate cancer with intent to cure are primarily surgery, radiation therapy, and proton therapy. Other treatments, such as hormonal therapy, chemotherapy, cryosurgery, and high intensity focused ultrasound (HIFU) also exist, depending on the clinical scenario and desired outcome.
The age and underlying health of the man, the extent of metastasis, appearance under the microscope, and response of the cancer to initial treatment are important in determining the outcome of the disease. The decision whether or not to treat localized prostate cancer (a tumor that is contained within the prostate) with curative intent is a patient trade-off between the expected beneficial and harmful effects in terms of patient survival and quality of life.
Contents |
Classification
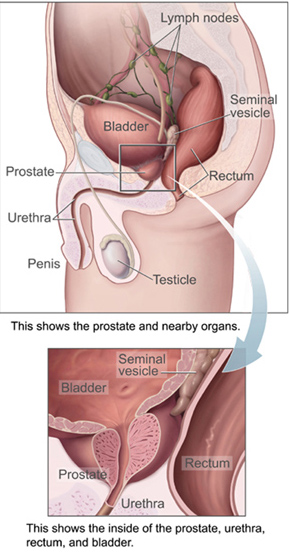
The prostate is a part of the male reproductive organ that helps make and store seminal fluid. In adult men, a typical prostate is about three centimeters long and weighs about twenty grams.[4] It is located in the pelvis, under the urinary bladder and in front of the rectum. The prostate surrounds part of the urethra, the tube that carries urine from the bladder during urination and semen during ejaculation.[5] Because of its location, prostate diseases often affect urination, ejaculation, and rarely defecation. The prostate contains many small glands which make about twenty percent of the fluid constituting semen.[6] In prostate cancer, the cells of these prostate glands mutate into cancer cells. The prostate glands require male hormones, known as androgens, to work properly. Androgens include testosterone, which is made in the testes; dehydroepiandrosterone, made in the adrenal glands; and dihydrotestosterone, which is converted from testosterone within the prostate itself. Androgens are also responsible for secondary sex characteristics such as facial hair and increased muscle mass.
An important part of evaluating prostate cancer is determining the stage, or how far the cancer has spread. Knowing the stage helps define prognosis and is useful when selecting therapies. The most common system is the four-stage TNM system (abbreviated from Tumor/Nodes/Metastases). Its components include the size of the tumor, the number of involved lymph nodes, and the presence of any other metastases.[7]
The most important distinction made by any staging system is whether or not the cancer is still confined to the prostate. In the TNM system, clinical T1 and T2 cancers are found only in the prostate, while T3 and T4 cancers have spread elsewhere. Several tests can be used to look for evidence of spread. These include computed tomography to evaluate spread within the pelvis, bone scans to look for spread to the bones, and endorectal coil magnetic resonance imaging to closely evaluate the prostatic capsule and the seminal vesicles. Bone scans should reveal osteoblastic appearance due to increased bone density in the areas of bone metastasis—opposite to what is found in many other cancers that metastasize.
Computed tomography (CT) and magnetic resonance imaging (MRI) currently do not add any significant information in the assessment of possible lymph node metastases in patients with prostate cancer according to a meta-analysis.[8] The sensitivity of CT was 42% and specificity of CT was 82%. The sensitivity of MRI was 39% and the specificity of MRI was 82%. For patients at similar risk to those in this study (17% had positive pelvic lymph nodes in the CT studies and 30% had positive pelvic lymph nodes in the MRI studies), this leads to a positive predictive value (PPV) of 32.3% with CT, 48.1% with MRI, and negative predictive value (NPV) of 87.3% with CT, 75.8% with MRI.
After a prostate biopsy, a pathologist looks at the samples under a microscope. If cancer is present, the pathologist reports the grade of the tumor. The grade tells how much the tumor tissue differs from normal prostate tissue and suggests how fast the tumor is likely to grow. The Gleason system is used to grade prostate tumors from 2 to 10, where a Gleason score of 10 indicates the most abnormalities. The pathologist assigns a number from 1 to 5 for the most common pattern observed under the microscope, then does the same for the second-most-common pattern. The sum of these two numbers is the Gleason score. The Whitmore-Jewett stage is another method sometimes used.
Signs and symptoms
Early prostate cancer usually causes no symptoms. Often it is diagnosed during the workup for an elevated PSA noticed during a routine checkup.
Sometimes, however, prostate cancer does cause symptoms, often similar to those of diseases such as benign prostatic hyperplasia. These include frequent urination, increased urination at night, difficulty starting and maintaining a steady stream of urine, blood in the urine, and painful urination. Prostate cancer is associated with urinary dysfunction as the prostate gland surrounds the prostatic urethra. Changes within the gland, therefore, directly affect urinary function. Because the vas deferens deposits seminal fluid into the prostatic urethra, and secretions from the prostate gland itself are included in semen content, prostate cancer may also cause problems with sexual function and performance, such as difficulty achieving erection or painful ejaculation.[9]
Advanced prostate cancer can spread to other parts of the body, possibly causing additional symptoms. The most common symptom is bone pain, often in the vertebrae (bones of the spine), pelvis, or ribs. Spread of cancer into other bones such as the femur is usually to the proximal part of the bone. Prostate cancer in the spine can also compress the spinal cord, causing leg weakness and urinary and fecal incontinence.[10]
Pathophysiology

Prostate cancer is classified as an adenocarcinoma, or glandular cancer, that begins when normal semen-secreting prostate gland cells mutate into cancer cells. The region of prostate gland where the adenocarcinoma is most common is the peripheral zone. Initially, small clumps of cancer cells remain confined to otherwise normal prostate glands, a condition known as carcinoma in situ or prostatic intraepithelial neoplasia (PIN). Although there is no proof that PIN is a cancer precursor, it is closely associated with cancer. Over time, these cancer cells begin to multiply and spread to the surrounding prostate tissue (the stroma) forming a tumor. Eventually, the tumor may grow large enough to invade nearby organs such as the seminal vesicles or the rectum, or the tumor cells may develop the ability to travel in the bloodstream and lymphatic system. Prostate cancer is considered a malignant tumor because it is a mass of cells that can invade other parts of the body. This invasion of other organs is called metastasis. Prostate cancer most commonly metastasizes to the bones, lymph nodes, rectum, and bladder.
The prostate is a zinc accumulating, citrate producing organ. The protein ZIP1 is responsible for the active transport of zinc into prostate cells. One of zinc's important roles is to change the metabolism of the cell in order to produce citrate, an important component of semen. The process of zinc accumulation, alteration of metabolism, and citrate production is energy inefficient, and prostate cells sacrifice enormous amounts of energy (ATP) in order to accomplish this task. Prostate cancer cells are generally devoid of zinc. This allows prostate cancer cells to save energy not making citrate, and utilize the new abundance of energy to grow and spread. The absence of zinc is thought to occur via a silencing of the gene that produces the transporter protein ZIP1. ZIP1 is now called a tumor suppressor gene product for the gene SLC39A1. The cause of the epigenetic silencing is unknown. Strategies which transport zinc into transformed prostate cells effectively eliminate these cells in animals. Zinc inhibits NF-κB pathways, is anti-proliferative, and induces apoptosis in abnormal cells. Unfortunately, oral ingestion of zinc is ineffective since high concentrations of zinc into prostate cells is not possible without the active transporter, ZIP1.[11]
RUNX2 is a transcription factor that prevents cancer cells from undergoing apoptosis thereby contributing to the development of prostate cancer.[12]
The PI3k/Akt signaling cascade works with the transforming growth factor beta/SMAD signaling cascade to ensure prostate cancer cell survival and protection against apoptosis.[13] X-linked inhibitor of apoptosis (XIAP) is hypothesized to promote prostate cancer cell survival and growth and is a target of research because if this inhibitor can be shut down then the apoptosis cascade can carry on its function in preventing cancer cell proliferation.[14] Macrophage inhibitory cytokine-1 (MIC-1) stimulates the focal adhesion kinase (FAK) signaling pathway which leads to prostate cancer cell growth and survival.[15]
The androgen receptor helps prostate cancer cells to survive and is a target for many anti cancer research studies; so far, inhibiting the androgen receptor has only proven to be effective in mouse studies.[16] Prostate specific membrane antigen (PSMA) stimulates the development of prostate cancer by increasing folate levels for the cancer cells to use to survive and grow; PSMA increases available folates for use by hydrolyzing glutamated folates.[17]
Screening
Prostate cancer screening is an attempt to find unsuspected cancers, and may lead to more specific follow-up tests such as a biopsy, with cell samples taken for closer study. Prostate cancer screening options include the digital rectal exam and the prostate-specific antigen (PSA) blood test. Prostate cancer is usually slow-growing and more common among older men. However, most cancers never grow enough to cause symptoms, and most men that have prostate cancer will never become aware of it in their lifetimes.
In Sweden, researchers recently found that prostate cancer really is "no longer a fatal disease." Even without treatment, researchers conclude that "only a small minority of men diagnosed with early-stage prostate cancer die from the disease," and that there is "no need to panic." Modern screening tests have found cancers that might never have developed into serious disease, and that "the slight reduction of risk by surgically removing the prostate or treating it with radiation may not outweigh the substantial side effects of these treatments," an opinion also shared by the CDC.[18][19]
Controversy over usage
Screening for prostate cancer is controversial because of cost and uncertain long-term benefits to patients.[20] Testing may lead to overdiagnosis and additional, but often unnecessary, testing and treatment. Follow-up tests can include painful biopsies which can result in excessive bleeding and infection. The discoverer of PSA, Dr. Richard J. Ablin, concludes that the test's popularity "has led to a hugely expensive public health disaster," as only 16 percent of men will ever receive a diagnosis of prostate cancer, but only a 3 percent chance of dying from it. He states that "the test is hardly more effective than a coin toss."[21] Dr. Horan echos that sentiment in his book.[22]
According to the American Urological Association, the controversy over prostate cancer should not surround the test, but rather how test results influence the decision to treat:
- "The decision to proceed to prostate biopsy should be based not only on elevated PSA and/or abnormal DRE results, but should take into account multiple factors including free and total PSA, patient age, PSA velocity, PSA density, family history, ethnicity, prior biopsy history and comorbidities.
- "A cancer cannot be treated if it is not detected. Not all prostate cancers require immediate treatment; active surveillance, in lieu of immediate treatment, is an option that should be considered for some men. Testing empowers patients and their urologists with the information to make an informed decision."[23]
In 2002, the U.S. Preventive Services Task Force concluded that "evidence was insufficient to recommend for or against screening." [24] Currently, the American Centers for Disease Control and Prevention (CDC), answers the question, "Should I Get Screened for Prostate Cancer?" with a statement:
- "Not all medical experts agree that screening for prostate cancer will save lives. Currently, there is not enough evidence to decide if the potential benefits of prostate cancer screening outweigh the potential risks."[18]
Private medical institutes, such as the Mayo Clinic, likewise acknowledge that "organizations vary in their recommendations about who should — and who shouldn't — get a PSA screening test." They conclude: "Ultimately, whether you should have a PSA test is something you'll have to decide after discussing it with your doctor, considering your risk factors and weighing your personal preferences."[25]
Unnecessary public expense
The annual cost of PSA screening in the U.S. totals at least $3 billion, with much of it paid for by Medicare and the Veterans Administration. A study in Europe resulted in only a small decline in death rates and concluded that 48 men would need to be treated to save one life. But of the 47 men who were treated, most would be unable to ever again function sexually and require more frequent trips to the bathroom.[21][22]
A study by the New England Journal of Medicine found that over a 7 to 10 year period, "screening did not reduce the death rate in men 55 and over."[21][22] Former screening proponents, including some from Stanford University, have come out against routine testing. In February 2010, the American Cancer Society urged "more caution in using the test." And the American College of Preventive Medicine concluded that "there was insufficient evidence to recommend routine screening."[21][22]
According to Ablin, "testing should absolutely not be deployed to screen the entire population of men over the age of 50 . . ." He concludes that the primary promoters of tests are drug companies, which "continue peddling the tests," along with advocacy groups including the American Urological Association, all of which "stand to profit" by pushing continual tests. He states:
- "I never dreamed that my discovery four decades ago would lead to such a profit-driven public health disaster. The medical community must confront reality and stop the inappropriate use of P.S.A. screening. Doing so would save billions of dollars and rescue millions of men from unnecessary, debilitating treatments."[21][22]
Diagnosis
The only test that can fully confirm the diagnosis of prostate cancer is a biopsy, the removal of small pieces of the prostate for microscopic examination. However, prior to a biopsy, several other tools may be used to gather more information about the prostate and the urinary tract. Digital rectal examination may allow a doctor to detect prostate abnormalities. Cystoscopy shows the urinary tract from inside the bladder, using a thin, flexible camera tube inserted down the urethra. Transrectal ultrasonography creates a picture of the prostate using sound waves from a probe in the rectum.
Biopsy
If cancer is suspected, a biopsy is offered expediently. During a biopsy a urologist or radiologist obtains tissue samples from the prostate via the rectum. A biopsy gun inserts and removes special hollow-core needles (usually three to six on each side of the prostate) in less than a second. Prostate biopsies are routinely done on an outpatient basis and rarely require hospitalization. Fifty-five percent of men report discomfort during prostate biopsy.[26]
Gleason score
The tissue samples are then examined under a microscope to determine whether cancer cells are present, and to evaluate the microscopic features (or Gleason score) of any cancer found. Prostate specific membrane antigen is a transmembrane carboxypeptidase and exhibits folate hydrolase activity.[27] This protein is overexpressed in prostate cancer tissues and is associated with a higher Gleason score.[27]
Tumor markers
Tissue samples can be stained for the presence of PSA and other tumor markers in order to determine the origin of malignant cells that have metastasized.[28]
Small cell carcinoma is a very rare (1%[29]) type of prostate cancer that cannot be diagnosed using the PSA.[30][29] As of 2009[update] researchers are trying to determine the best way to screen for this type of prostate cancer because it is a relatively unknown and rare type of prostate cancer but very serious and quick to spread to other parts of the body.[30] Possible methods include chromatographic separation methods by mass spectrometry, or protein capturing by immunoassays or immunized antibodies. The test method will involve quantifying the amount of the biomarker PCI, with reference to the Gleason Score. Not only is this test quick, it is also sensitive. It can detect patients in the diagnostic grey zone, particularly those with a serum free to total Prostate Specific Antigen ratio of 10-20%.[31]
The oncoprotein BCL-2, has been associated with the development of androgen-independent prostate cancer due to its high levels of expression in androgen-independent tumours in advanced stages of the pathology. The upregulation of BCL-2 after androgen ablation in prostate carcinoma cell lines and in a castrated-male rat model further established a connection between BCL-2 expression and prostate cancer progression.[32]
The expression of Ki-67 by immunohistochemistry may be a significant predictor of patient outcome for men with prostate cancer.[33]
Diagnostic tools under investigation
At present, an active area of research involves non-invasive methods of prostate tumor detection. Adenoviruses modified to transfect tumor cells with harmless yet distinct genes (such as luciferase) have proven capable of early detection. So far, however, this area of research has been tested only in animal and LNCaP cell models.[34]
PCA3
Another potential non-invasive method of early prostate tumor detection is through a molecular test that detects the presence of cell-associated PCA3 mRNA in urine. PCA3 mRNA is expressed almost exclusively by prostate cells and has been shown to be highly over-expressed in prostate cancer cells. PCA3 is not a replacement for PSA but an additional tool to help decide whether, in men suspected of having prostate cancer, a biopsy is really needed. The higher the expression of PCA3 in urine, the greater the likelihood of a positive biopsy, i.e., the presence of cancer cells in the prostate.
Early prostate cancer antigen-2
It was reported in April 2007 that a new blood test for early prostate cancer antigen-2 (EPCA-2) that may alert men if they have prostate cancer and how aggressive it will be is being researched.[35][36]
Thrombophlebitis is associated with an increased risk of prostate cancer and may be a good way for physicians to remind themselves to screen patients with thrombophlebitis for prostate cancer as well since these two are closely linked.[37]
Prostate mapping
Prostate mapping is a method of diagnosis that may be accurate in determining the precise location and aggressiveness of a tumor. It uses a combination of multi-sequence MRI imaging techniques and a template-guided biopsy system, and involves taking multiple biopsies through the skin that lies in front of the rectum rather than through the rectum itself. The procedure is carried out under general anesthetic.[38]
Prostasomes
Epithelial cells of the prostate secrete prostasomes as well as PSA. Prostasomes are membrane–surrounded, prostate-derived organelles that appear extracellularly, and one of their physiological functions is to protect the sperm from attacks by the female immune system. Cancerous prostate cells continue to synthesize and secrete prostasomes, and may be shielded against immunological attacks by these prostasomes. Research of several aspects of prostasomal involvement in prostate cancer has been performed.[39]
Management
Treatment for prostate cancer may involve active surveillance (monitoring for tumor progress or symptoms), surgery (i.e. radical prostatectomy), radiation therapy including brachytherapy (prostate brachytherapy) and external beam radiation therapy, High-intensity focused ultrasound (HIFU), chemotherapy, oral chemotherapeutic drugs (Temozolomide/TMZ), positron emission tomography, cryosurgery, hormonal therapy, or some combination.[40][41][42]
Which option is best depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors are the man's age, his general health, and his feelings about potential treatments and their possible side-effects. Because all treatments can have significant side-effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations. Prostate cancer patients are strongly recommended to work closely with their urologist and use a combination of the treatment options when managing their prostate cancer.[43][44][45]
The selection of treatment options may be a complex decision involving many factors. For example, radical prostatectomy after primary radiation failure is a very technically challenging surgery and may not be an option.[46] This may enter into the treatment decision.
If the cancer has spread beyond the prostate, treatment options significantly change, so most doctors that treat prostate cancer use a variety of nomograms to predict the probability of spread. Treatment by watchful waiting/active surveillance, HIFU, external beam radiation therapy, brachytherapy, cryosurgery, and surgery are, in general, offered to men whose cancer remains within the prostate. Hormonal therapy and chemotherapy are often reserved for disease that has spread beyond the prostate. However, there are exceptions: radiation therapy may be used for some advanced tumors, and hormonal therapy is used for some early stage tumors. Cryotherapy (the process of freezing the tumor), hormonal therapy, and chemotherapy may also be offered if initial treatment fails and the cancer progresses.[47]
Dr. Aaron Katz studied the results of combining conventional treatment with holistic therapies.[48]
Prostate cancer can be treated by using Ad.DD3-E1A-IL-24 which stimulates apoptosis in cancer cells thereby inhibiting tumor growth.[49]
Hormone-refractory prostate cancer (HRPC)
Most hormone dependent cancers become refractory (independent) after one to three years and resume growth despite hormone therapy.
Docetaxel has been used for HRPC with a median survival benefit of 2 to 3 months.[50][51]
A combination of bevacizumab(Avastin), taxotere, thalidomide and prednisone appears effective in the treatment of hormone-refractory prostate cancer.[52]
Provenge is also effective (better than placebo) in the treatment of hormone-refractory prostate cancer[53], as is Cabazitaxel [54].
Abiraterone showed good results in a phase 2 trial and As of 2009[update] is in two phase 3 clinical trials.
Prognosis
Prostate cancer rates are higher and prognosis poorer in developed countries than the rest of the world. Many of the risk factors for prostate cancer are more prevalent in the developed world, including longer life expectancy and diets high in red meat (People that consume larger amounts of meat and dairy also tend to consume fewer portions of fruits and vegetables. It is not currently clear whether both of these factors, or just one of them, contribute to the occurrence of prostate cancer.[55]) Also, where there is more access to screening programs, there is a higher detection rate. Prostate cancer is the ninth-most-common cancer in the world, but is the number-one non-skin cancer in United States men. Prostate cancer affected eighteen percent of American men and caused death in three percent in 2005.[56] In Japan, death from prostate cancer was one-fifth to one-half the rates in the United States and Europe in the 1990s.[57] In India in the 1990s, half of the people with prostate cancer confined to the prostate died within ten years.[58] African-American men have 50–60 times more prostate cancer and prostate cancer deaths than men in Shanghai, China.[59] In Nigeria, two percent of men develop prostate cancer and 64% of them are dead after two years.[60]
In patients that undergo treatment, the most important clinical prognostic indicators of disease outcome are stage, pre-therapy PSA level and Gleason score. In general, the higher the grade and the stage the poorer the prognosis. Nomograms can be used to calculate the estimated risk of the individual patient. The predictions are based on the experience of large groups of patients suffering from cancers at various stages.[61]
In 1941, Charles Huggins reported that androgen ablation therapy causes regression of primary and metastatic androgen-dependent prostate cancer.[62] Androgen ablation therapy causes remission in 80-90% of patients undergoing therapy, resulting in a median progression-free survival of 12 to 33 months. After remission, an androgen-independent phenotype typically emerges, wherein the median overall survival is 23–37 months from the time of initiation of androgen ablation therapy.[63] The actual mechanism contributes to the progression of prostate cancer is not clear and may vary between individual patient. A few possible mechanisms have been proposed.[64]
Classification systems
Many prostate cancers are not destined to be lethal, and most men will ultimately die from causes other than of the disease. Decisions about treatment type and timing may, therefore, be informed by an estimation of the risk that the tumor will ultimately recur after treatment and/or progress to metastases and mortality. Several tools are available to help predict outcomes such as pathologic stage and recurrence after surgery or radiation therapy. Most combine stage, grade, and PSA level, and some also add the number or percent of biopsy cores positive, age, and/or other information.
- The D'Amico classification stratifies men by low, intermediate, or high risk based on stage, grade, and PSA. It is used widely in clinical practice and research settings. The major downside to the 3-level system is that it does not account for multiple adverse parameters (e.g., high Gleason score and high PSA) in stratifying patients.
- The Partin tables predict pathologic outcomes (margin status, extraprostatic extension, and seminal vesicle invasion) based on the same 3 variables, and are published as lookup tables.
- The Kattan nomograms predict recurrence after surgery and/or radiation therapy, based on data available either at time of diagnosis or after surgery. The nomograms can be calculated using paper graphs, or using software available on a website or for handheld computers. The Kattan score represents the likelihood of remaining free of disease at a given time interval following treatment.
- The UCSF Cancer of the Prostate Risk Assessment (CAPRA) score predicts both pathologic status and recurrence after surgery. It offers comparable accuracy as the Kattan preoperative nomogram, and can be calculated without paper tables or a calculator. Points are assigned based on PSA, Grade, stage, age, and percent of cores positive; the sum yields a 0–10 score, with every 2 points representing roughly a doubling of risk of recurrence. The CAPRA score was derived from community-based data in the CaPSURE database. It has been validated among over 10,000 prostatectomy patients, including patients from CaPSURE[65]; the SEARCH registry, representing data from several Veterans Administration and active military medical centers[66]; a multi-institutional cohort in Germany[67]; and the prostatectomy cohort at Johns Hopkins University.[68] More recently, it has been shown to predict metastasis and mortality following prostatectomy, radiation therapy, watchful waiting, or androgen deprivation therapy.[69]
Epidemiology

Rates of prostate cancer vary widely across the world. Although the rates vary widely between countries, it is least common in South and East Asia, more common in Europe, and most common in the United States.[2] According to the American Cancer Society, prostate cancer is least common among Asian men and most common among black men, with figures for white men in between.[71][72] However, these high rates may be affected by increasing rates of detection.[73]
Prostate cancer develops primarily in men over fifty. It is the most common type of cancer in men in the United States, with 186,000 new cases in 2008 and 28,600 deaths.[74] It is the second leading cause of cancer death in U.S. men after lung cancer. In the United Kingdom it is also the second most common cause of cancer death after lung cancer, where around 35,000 cases are diagnosed every year and of which around 10,000 die of it. Many factors, including genetics and diet, have been implicated in the development of prostate cancer. The Prostate Cancer Prevention Trial found that finasteride reduces the incidence of prostate cancer rate by 30%. There had been a controversy about this also increasing the risk of more aggressive cancers, but more recent research showed this may not be the case.[75][76]
Causes
The specific causes of prostate cancer remain unknown.[77] The primary risk factors are age and family history. Prostate cancer is very uncommon in men younger than 45, but becomes more common with advancing age. The average age at the time of diagnosis is 70.[78] However, many men never know they have prostate cancer. Autopsy studies of Chinese, German, Israeli, Jamaican, Swedish, and Ugandan men who died of other causes have found prostate cancer in thirty percent of men in their 50s, and in eighty percent of men in their 70s.[79] Men who have first-degree family members with prostate cancer appear to have double the risk of getting the disease compared to men without prostate cancer in the family.[80] This risk appears to be greater for men with an affected brother than for men with an affected father. In the United States in 2005, there were an estimated 230,000 new cases of prostate cancer and 30,000 deaths due to prostate cancer.[81] Men with high blood pressure are more likely to develop prostate cancer.[82]A 2010 study found that prostate basal cells were the most common site of origin for prostate cancers.[83]
Genetics
Genetic background may contribute to prostate cancer risk, as suggested by associations with race, family, and specific gene variants. Men with one first-degree relatives with prostate cancer have a twofold higher risk, and those with two first-degree relatives have a fivefold greater risk of developing prostate cancer compared with men with no family history. In the United States, prostate cancer more commonly affects black men than white or Hispanic men, and is also more deadly in black men.[84] [85] In contrast, the incidence and mortality rates for Hispanic men are one third lower than for non-Hispanic whites. Men who have a brother or father with prostate cancer have twice the risk of developing prostate cancer.[86] Studies of twins in Scandinavia suggest that forty percent of prostate cancer risk can be explained by inherited factors.[87]
No single gene is responsible for prostate cancer; many different genes have been implicated. Mutations in BRCA1 and BRCA2, important risk factors for ovarian cancer and breast cancer in women, have also been implicated in prostate cancer.[88] Other linked genes include the Hereditary Prostate cancer gene 1 (HPC1), the androgen receptor, and the vitamin D receptor.[84] TMPRSS2-ETS gene family fusion, specifically TMPRSS2-ERG or TMPRSS2-ETV1/4 promotes cancer cell growth.[89]
Loss of cancer suppressor genes,early in the prostatic carcinogenesis, have been localized to chromosomes 8p, 10q, 13q,and 16q. P53 mutations in the primary prostate cancer are relatively low and are more frequently seen in Metastatic settings,hence, p53 mutations are late event in pathology of prostate cancer. Other tumor suppressor genes that are thought to play a role in prostate cancer include PTEN (gene) and KAI1. "Up to 70 percent of men with prostate cancer have lost one copy of the PTEN gene at the time of diagnosis"[90] Relative frequency of loss of E-cadherin and CD44 has also been observed.
Diet
Evidence from epidemiological studies supports a possible protective role in reducing prostate cancer for dietary Vitamin B6,[91] selenium, vitamin E, lycopene, and soy foods. A study in 2007 cast doubt on the effectiveness of lycopene (found in tomatoes) in reducing the risk of prostate cancer.[92] Lower blood levels of vitamin D may increase the risk of developing prostate cancer.[93] This may be linked to lower exposure to ultraviolet (UV) light, since UV light exposure can increase vitamin D in the body.[94]
Studies comparing men who live in areas of the country with high levels of selenium to men in areas with low levels suggest that this mineral protects against prostate cancer.[95] Selenium is believed to reduce the risk of developing prostate cancer because it keeps cells from proliferating or dying off in a rapid or unusual way. An analysis in 2002 of the Nutritional Prevention of Cancer Trial revealed that the men who took selenium supplements daily were half as likely to be diagnosed with prostate cancer.[96] These findings have been confirmed in most observational studies.[97] However, in 2008, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) indicated that neither selenium nor vitamin E, alone or in combination, was effective for the primary prevention of prostate cancer.[98][99][100] Whether or not selenium helps prevent prostate cancer, researchers at the Dana-Farber Cancer Institute in Boston found that higher selenium levels in the blood may worsen prostate cancer in many men who already have the disease.[100][101]
Green tea may be protective (due to its polyphenol content),[102] although the most comprehensive clinical study indicates that it has no protective effect.[103] Other holistic methods are also studied.[48]
Research published in the Journal of the National Cancer Institute suggests that taking multivitamins more than seven times a week can increase the risks of contracting the disease.[104][105] This research was unable to highlight the exact vitamins responsible for this increase (almost double), although they suggest that vitamin A, vitamin E and beta-carotene may lie at its heart. It is advised that those taking multivitamins never exceed the stated daily dose on the label.
A 2007 study published in the Journal of the National Cancer Institute found that men eating cauliflower, broccoli, or one of the other cruciferous vegetables, more than once a week were 40% less likely to develop prostate cancer than men who rarely ate those vegetables.[106][107] The phytochemicals indole-3-carbinol and diindolylmethane, found in cruciferous vegetables, has antiandrogenic and immune modulating properties.[108][109]
Many doctors prescribe supplements to prostate cancer patients but currently the efficacy of nutrient supplements is still unknown.[110] Supplements may not be as beneficial to prostate health as micronutrients obatined naturally from the diet.[110]
Folic acid supplements have recently been linked to an increase in risk of developing prostate cancer.[27] A ten-year research study led by University of Southern California researchers showed that men who took daily folic acid supplements of 1 mg were three times more likely to be diagnosed with prostate cancer than men who took a placebo.[27] Folate plays a complex role in prostate cancer and folic acid supplements have a different effect on prostate cancer than folate naturally found in foods.[27] The supplement form, folic acid, is more bioavailable in the body compared with dietary sources of folate.[27] Folate hydrolase activity is associated with prostate-specific antigen. A small Swedish study of 254 subjects, with a median age of 64, and a follow up of 5 years suggested that folate status is not protective against prostate cancer, however, and like folic acid may even result in a 3 fold increase in early prostate cancer development and risk.[111] Supplements and multivitamins, alcohol and drug consumption, GI disorders, and folate bioavailability were not analyzed in this study.[111]
High alcohol intake may increase the risk of prostate cancer and interfere with folate metabolism.[112] Low folate intake and high alcohol intake may increase the risk of prostate cancer to a greater extent than the sole effect of either one by itself.[112] A case control study consisting of 137 veterans addressed this hypothesis and the results were that high folate intake was related to a 79% lower risk of developing prostate cancer and there was no association between alcohol consumption by itself and prostate cancer risk.[112] Folate's effect however was only significant when coupled with low alcohol intake.[112] There is a significant decrease in risk of prostate cancer with increasing dietary folate intake but this association only remains in individuals with low levels of alcohol consumption.[112] There was no association found in this study between folic acid supplements and risk of prostate cancer.[112]
The prostate gland has a high concentration of zinc so zinc may play a role in prostate cancer.[113] Researchers studied the relationship between zinc supplement intake of 100 mg/day and the risk of prostate cancer in 46 974 US men over a 14 year period and reported in 2003 that long term zinc supplement of over 100mg/day intake seemed to be associated with approximately double the risk of developing prostate cancer.[113]
Medication exposure
There are also some links between prostate cancer and medications, medical procedures, and medical conditions. Daily use of anti-inflammatory medicines such as aspirin, ibuprofen, or naproxen may decrease prostate cancer risk.[114] Use of the cholesterol-lowering drugs known as the statins may also decrease prostate cancer risk.[115]
Infection or inflammation of the prostate (prostatitis) may increase the chance for prostate cancer. In particular, infection with the sexually transmitted infections chlamydia, gonorrhea, or syphilis seems to increase risk.[116] Finally, obesity[117] and elevated blood levels of testosterone[118] may increase the risk for prostate cancer. There is an association between vasectomy and prostate cancer however more research is needed to determine if this is a causative relationship.[119]
Research released in May 2007, found that US war veterans who had been exposed to Agent Orange had a 48% increased risk of prostate cancer recurrence following surgery.[120]
Potential viral cause
In 2006, researchers associated a previously unknown retrovirus, Xenotropic MuLV-related virus or XMRV, with human prostate tumors.[121] Subsequent reports on the virus have been contradictory. A group of US researchers found XMRV protein expression in human prostate tumors,[122] while German scientists failed to find XMRV-specific antibodies or XMRV-specific nucleic acid sequences in prostate cancer samples.[123]
Prevention
A comprehensive worldwide report Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective compiled by the World Cancer Research Fund and the American Institute for Cancer Research reports a significant relation between lifestyle (including food consumption) and cancer prevention. Other research also supports this finding.[110] Exercise and diet may help prevent prostate cancer to the same extent as medications such as alpha-blockers and 5-alpha-reductase inhibitors.[110] The potential role of diet in preventing prostate cancer is discussed in greater detail in the diet section of this article.
Two medications which block the conversion of testosterone to dihydrotestosterone, finasteride[124] and dutasteride,[125] have also shown some promise. The use of these medications for primary prevention is still in the testing phase, and they are not widely used for this purpose. A 2008 study found that finasteride reduces the incidence of prostate cancer by 30%, without any increase in the risk of High-Grade prostate cancer.[75] In the original study it turns out that the smaller prostate caused by finasteride means that a doctor is more likely to hit upon cancer nests and more likely to find aggressive-looking cells.[75]
Ejaculation frequency
More frequent ejaculation also may decrease a man's risk of prostate cancer. One study showed that men who ejaculated five times a week in their 20s had a decreased rate of prostate cancer, though other studies have shown no benefit.[126][127] The results contradict those of previous studies, which have suggested that having had many sexual partners, or a high frequency of sexual activity, increases the risk of prostate cancer by up to 40 percent. A key difference may be that these earlier studies defined sexual activity as sexual intercourse, whereas this study focused on the number of ejaculations, whether or not intercourse was involved.[128] Another study completed in 2004 reported that "Most categories of ejaculation frequency were unrelated to risk of prostate cancer. However, high ejaculation frequency was related to decreased risk of total prostate cancer." The report abstract concluded, "Our results suggest that ejaculation frequency is not related to increased risk of prostate cancer."[129]
Diet
Oils and fatty acids
In experimental models using mice have been tested, dietary and serum omega-6 polyunsaturated fatty acids (PUFAs) increased prostate tumor growth,and has sped up histopathological progression, and decreased survival, while the omega-3 fatty acids, in the same situation, had the opposite, beneficial effect.[130]
Men with high serum linoleic acid, but not palmitic, can reduce the risk of prostate cancer by taking tocopherol supplementation.[131]
Men with elevated levels of long-chain omega-3 fatty acids (EPA and DHA) had lowered incidence.[132]
A long-term study reports that "blood levels of trans fatty acids, in particular trans fats resulting from the hydrogenation of vegetable oils, are associated with an increased prostate cancer risk."[133]
Some researchers have indicated that serum myristic acid[131][134] and palmitic acid[134] and dietary myristic[135] and palmitic[135] saturated fatty acids and serum palmitic combined with alpha-tocopherol supplementation[131] are associated with increased risk of prostate cancer in a dose-dependent manner. These associations may, however, reflect differences in intake or metabolism of these fatty acids between the precancer cases and controls, rather than being an actual cause.[134]
Other
Vegetarian: The American Dietetic Association and Dieticians of Canada report a decreased incidence of prostate cancer for those following a vegetarian diet.[136]
Low-carb : In lab tests on mice, prostate tumors grow slower with a no-carbohydrate diet.[137]
Coffee: A preliminary study found a correlation between coffee consumption and a lower risk of aggressive prostate cancer.[138]
History
Although the prostate was first described by Venetian anatomist Niccolò Massa in 1536, and illustrated by Flemish anatomist Andreas Vesalius in 1538, prostate cancer was not identified until 1853.[139] Prostate cancer was initially considered a rare disease, probably because of shorter life expectancies and poorer detection methods in the 19th century. The first treatments of prostate cancer were surgeries to relieve urinary obstruction.[140] Removal of the entire gland (radical perineal prostatectomy) was first performed in 1904 by Hugh H. Young at Johns Hopkins Hospital.[141] Surgical removal of the testes (orchiectomy) to treat prostate cancer was first performed in the 1890s, but with limited success. Transurethral resection of the prostate (TURP) replaced radical prostatectomy for symptomatic relief of obstruction in the middle of the 20th century because it could better preserve penile erectile function. Radical retropubic prostatectomy was developed in 1983 by Patrick Walsh.[142] This surgical approach allowed for removal of the prostate and lymph nodes with maintenance of penile function.
In 1941, Charles B. Huggins published studies in which he used estrogen to oppose testosterone production in men with metastatic prostate cancer. This discovery of "chemical castration" won Huggins the 1966 Nobel Prize in Physiology or Medicine.[143] The role of the hormone GnRH in reproduction was determined by Andrzej W. Schally and Roger Guillemin, who both won the 1977 Nobel Prize in Physiology or Medicine for this work.
Receptor agonists, such as leuprolide and goserelin, were subsequently developed and used to treat prostate cancer.[144][145]
Radiation therapy for prostate cancer was first developed in the early 20th century and initially consisted of intraprostatic radium implants. External beam radiation became more popular as stronger radiation sources became available in the middle of the 20th century. Brachytherapy with implanted seeds was first described in 1983.[146]
Systemic chemotherapy for prostate cancer was first studied in the 1970s. The initial regimen of cyclophosphamide and 5-fluorouracil was quickly joined by multiple regimens using a host of other systemic chemotherapy drugs.[147]
On 30 July, 2010 Owen Witte M.D. et al. of UCLA published a series of studies in Science during which they had introduced viruses known to cause cancerous mutation in prostate cells: AKT, ERG, and AR into isolated samples of basal and luminal cells and grafted the treated tissue into mice. After 16 weeks, none of the luminal samples had undergone malignant mutation, while the basal samples had mutated into prostate-like tubules which had then developed malignancy and formed cancerous tumors, which appeared identical to human samples under magnification. This led to the conclusion that the prostate basal cell may be the most likely "site of origin" of prostate cancer. [148]
Research
Androgen at a concentration of 10-fold higher than the physiological concentration has also been shown to cause growth suppression and reversion of androgen-independent prostate cancer xenografts or androgen-independent prostate tumors derived in vivo model to an androgen-stimulated phenotype in athymic mice.[149][150] These observation suggest the possibility to use androgen to treat the development of relapsed androgen-independent prostate tumors in patients.
Oral infusion of green tea polyphenols, a potential alternative therapy for prostate cancer by natural compounds, has been shown to inhibit the development, progression, and metastasis as well in autochthonous transgenic adenocarcinoma of the mouse prostate (TRAMP) model, which spontaneously develops prostate cancer.[151]
The insulin-like growth factor signaling axis is thought to play a key role in the progression of prostate carcinoma. It consists of two ligands (IGF-1 and IGF-2), two receptors (IGF-IR and IGF-IIR) and six related high-affinity IGF-binding proteins (IGFBP 1-6).[152] Altered expression of IGF axis members has been implicated in the development of many different types of cancers, including prostate.[153][154]
The Color of Prostate Cancer Awareness is Light Blue.
Prostate cancer models
Scientists have established a few prostate cancer cell lines to investigate the mechanism involved in the progression of prostate cancer. LNCaP, PC-3 (PC3), and DU-145 (DU145) are commonly used prostate cancer cell lines. The LNCaP cancer cell line was established from a human lymph node metastatic lesion of prostatic adenocarcinoma. PC-3 and DU-145 cells were established from human prostatic adenocarcinoma metastatic to bone and to brain, respectively. LNCaP cells express androgen receptor (AR); however, PC-3 and DU-145 cells express very little or no AR. AR, an androgen-activated transcription factor, belongs to the steroid nuclear receptor family. Development of the prostate is dependent on androgen signaling mediated through AR, and AR is also important during the development of prostate cancer. The proliferation of LNCaP cells is androgen-dependent but the proliferation of PC-3 and DU-145 cells is androgen-insensitive. Elevation of AR expression is often observed in advanced prostate tumors in patients.[155][156] Some androgen-independent LNCaP sublines have been developed from the ATCC androgen-dependent LNCaP cells after androgen deprivation for study of prostate cancer progression. These androgen-independent LNCaP cells have elevated AR expression and express prostate specific antigen upon androgen treatment. The paradox is that androgens inhibit the proliferation of these androgen-independent prostate cancer cells.[157][158][159]
See also
- Dennis Hopper (American director/actor)
- Frank Zappa (American composer)
References
- ↑ "ACS :: What Is Prostate Cancer?" American Cancer Society :: Information and Resources for Cancer: Breast, Colon, Prostate, Lung and Other Forms. Web. 15 June 2010. "?". http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_is_prostate_cancer_36.asp?sitearea=. Retrieved 9 August 2010.
- ↑ 2.0 2.1 "IARC Worldwide Cancer Incidence Statistics—Prostate". JNCI Cancer Spectrum. Oxford University Press. December 19, 2001. http://web.archive.org/web/20060205235509/http://www.jncicancerspectrum.oxfordjournals.org/cgi/statContent/cspectfstat;99. Retrieved on 5 April 2007 through the Internet Archive
- ↑ Sam Lister (February 11, 2009). "Urine test could speed treatment of prostate cancer". The Sunday Times. http://www.timesonline.co.uk/tol/news/uk/health/article5710450.ece. Retrieved 9 August 2010.
- ↑ Aumüller, G. (1979). Prostate Gland and Seminal Vesicles. Berlin-Heidelberg: Springer-Verlag.
- ↑ Moore, K.; Dalley, A. (1999). Clinically Oriented Anatomy. Baltimore, Maryland: Lippincott Williams & Wilkins. ISBN 0683061321.
- ↑ Steive, H. (1930). "Männliche Genitalorgane". Handbuch der mikroskopischen Anatomie des Menschen. Vol. VII Part 2. Berlin: Springer. pp. 1–399.
- ↑ BMJ Group (8 December 2009). "Prostate cancer: How far has your cancer spread? The TNM system". Guardian.co.uk. http://www.guardian.co.uk/lifeandstyle/besttreatments/prostate-cancer-how-far-has-your-cancer-spread-the-tnm-system. Retrieved 9 August 2010.
- ↑ Smith JA, Chan RC, Chang SS, et al. (2007). "A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy". J. Urol. 178 (6): 2385–9; discussion 2389–90. doi:10.1016/j.juro.2007.08.008. PMID 17936849.
- ↑ Miller DC, Hafez KS, Stewart A, Montie JE, Wei JT (September 2003). "Prostate carcinoma presentation, diagnosis, and staging: an update form the National Cancer Data Base". Cancer 98 (6): 1169–78. doi:10.1002/cncr.11635. PMID 12973840.
- ↑ van der Cruijsen-Koeter, IW; Vis AN, Roobol MJ, Wildhagen MF, de Koning HJ, van der Kwast TH, Schroder FH (July 2005). "Comparison of screen detected and clinically diagnosed prostate cancer in the European randomized study of screening for prostate cancer, section rotterdam". Urol 174 (1): 121–5. doi:10.1097/01.ju.0000162061.40533.0f. PMID 15947595.
- ↑ Journal-molecular cancer, review, 2006 5:17, doi:10.1186/1476-4598-5-17
- ↑ Leav I, Plescia J, Goel HL, Li J, Jiang Z, Cohen RJ, Languino LR, Altieri DC (January 2010). "Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer". Am. J. Pathol. 176 (1): 393–401. doi:10.2353/ajpath.2010.090521. PMID 19948822.
- ↑ Zha J, Huang YF (September 2009). "[TGF-beta/Smad in prostate cancer: an update]" (in Chinese). Zhonghua Nan Ke Xue 15 (9): 840–3. PMID 19947572.
- ↑ Watanabe SI, Miyata Y, Kanda S, Iwata T, Hayashi T, Kanetake H, Sakai H (November 2009). "Expression of X-linked inhibitor of apoptosis protein in human prostate cancer specimens with and without neo-adjuvant hormonal therapy". J Cancer Res Clin Oncol 136 (5): 787–93. doi:10.1007/s00432-009-0718-x. PMID 19946707.
- ↑ Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK (March 2010). "Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway". Oncogene 29 (9): 1293–302. doi:10.1038/onc.2009.420. PMID 19946339.
- ↑ Narizhneva NV, Tararova ND, Ryabokon P, Shyshynova I, Prokvolit A, Komarov PG, Purmal AA, Gudkov AV, Gurova KV (December 2009). "Small molecule screening reveals a transcription-independent pro-survival function of androgen receptor in castration-resistant prostate cancer". Cell Cycle 8 (24): 4155–67. PMID 19946220.
- ↑ Yao V, Berkman CE, Choi JK, O'Keefe DS, Bacich DJ (February 2010). "Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid". Prostate 70 (3): 305–16. doi:10.1002/pros.21065. PMID 19830782.
- ↑ 18.0 18.1 Prostate Cancer Screening CDC, updated April 6, 2010
- ↑ "Untreated prostate cancer no death sentence", Reuters, June 18, 2010
- ↑ Collins MM, Barry MJ (December 1996). "Controversies in prostate cancer screening. Analogies to the early lung cancer screening debate". JAMA 276 (24): 1976–9. doi:10.1001/jama.276.24.1976. PMID 8971068.
- ↑ 21.0 21.1 21.2 21.3 21.4 Ablin RJ (2010-03-09). "The Great Prostate Mistake". The New York Times. http://www.nytimes.com/2010/03/10/opinion/10Ablin.html.
- ↑ 22.0 22.1 22.2 22.3 22.4 [|Horan, Anthony] (31 Aug 2009). The Big Scare; the Business of Prostate Cancer. SterlingHouse. ISBN 1585011193.
- ↑ "AUA clarifes recommendations on prostate cancer testing with PSA test and DRE" The Medical News, Nov. 4, 2009
- ↑ Lin K, Lipsitz R, Miller T, Janakiraman S (August 2008). "Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force". Ann. Intern. Med. 149 (3): 192–9. PMID 18678846. http://www.ahrq.gov/clinic/uspstf08/prostate/prostateart.pdf.
- ↑ "Prostate cancer screening: Should you get a PSA test?" MayoClinic.com, updated March 6, 2010
- ↑ Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schröder FH (June 1998). "Short-term effects of population-based screening for prostate cancer on health-related quality of life". J. Natl. Cancer Inst. 90 (12): 925–31. doi:10.1093/jnci/90.12.925. PMID 9637143.
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, Burke CA, McKeown-Eyssen GE, Baron JA (March 2009). "Folic acid and risk of prostate cancer: results from a randomized clinical trial". J. Natl. Cancer Inst. 101 (6): 432–5. doi:10.1093/jnci/djp019. PMID 19276452.
- ↑ Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI (August 2007). "Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma". Am. J. Surg. Pathol. 31 (8): 1246–55. doi:10.1097/PAS.0b013e31802f5d33. PMID 17667550.
- ↑ 29.0 29.1 Nutting C, Horwich A, Fisher C, Parsons C, Dearnaley DP (June 1997). "Small-cell carcinoma of the prostate". Journal of the Royal Society of Medicine 90 (6): 340–1. PMID 9227387.
- ↑ 30.0 30.1 Wei ZF, Xu H, Wang H, Wei W, Cheng W, Zhou WQ, Ge JP, Zhang ZY, Gao JP, Yin HL (September 2009). "[Clinicopathological characterization of prostatic small cell carcinoma: a case report and review of the literature]" (in Chinese). Zhonghua Nan Ke Xue 15 (9): 829–32. PMID 19947569.
- ↑ [1]
- ↑ Catz SD, Johnson JL (January 2003). "BCL-2 in prostate cancer: a minireview". Apoptosis 8 (1): 29–37. doi:10.1023/A:1021692801278. PMID 12510149.
- ↑ Srikumar Chakravarthi, David Low Wee Yang, Thanikachalam P, Nagaraja HS, Nadeem Irfan Bukhari (2009). "Assessment of proliferative index and its association with Ki-67 antigen molecule expression in nodular hyperplasia of prostate". Indian Journal of Science & Technology 2 (8): 1–4. ISSN 0974-6846.
- ↑ Iyer M, Salazar FB, Lewis X, et al. (February 2005). "Non-invasive imaging of a transgenic mouse model using a prostate-specific two-step transcriptional amplification strategy". Transgenic Res. 14 (1): 47–55. doi:10.1007/s11248-004-2836-1. PMID 15865048. http://www.springerlink.com/openurl.asp?genre=article&issn=0962-8819&volume=14&issue=1&spage=47.
- ↑ A Prostate Cancer Revolution. Newsweek, April 26, 2007.
- ↑ Hansel DE, DeMarzo AM, Platz EA, Jadallah S, Hicks J, Epstein JI, Partin AW, Netto GJ (May 2007). "Early prostate cancer antigen expression in predicting presence of prostate cancer in men with histologically negative biopsies". J. Urol. 177 (5): 1736–40. doi:10.1016/j.juro.2007.01.013. PMID 17437801.
- ↑ van Weert HC, Pingen F (2009). "Recurrent thromboflebitis as a warning sign for cancer: a case report". Cases J 2: 153. doi:10.1186/1757-1626-2-153. PMID 19946524.
- ↑ Sartor AO, Hricak H, Wheeler TM, Coleman J, Penson DF, Carroll PR, Rubin MA, Scardino PT (December 2008). "Evaluating localized prostate cancer and identifying candidates for focal therapy". Urology 72 (6 Suppl): S12–24. doi:10.1016/j.urology.2008.10.004. PMID 19095124.
- ↑ Nilsson BO, Carlsson L, Larsson A, Ronquist G (2001). "Autoantibodies to prostasomes as new markers for prostate cancer". Ups. J. Med. Sci. 106 (1): 43–9. doi:10.3109/2000-1967-171. PMID 11817562.
- ↑ Hong H, Zhang Y, Sun J, Cai W (November 2009). "Positron emission tomography imaging of prostate cancer". Amino Acids 39 (1): 11–27. doi:10.1007/s00726-009-0394-9. PMID 19946787.
- ↑ Braun K, Ehemann V, Wiessler M, Pipkorn R, Didinger B, Mueller G, Waldeck W (2009). "High-resolution flow cytometry: a suitable tool for monitoring aneuploid prostate cancer cells after TMZ and TMZ-BioShuttle treatment". Int J Med Sci 6 (6): 338–47. PMID 19946604.
- ↑ Peyromaure M, Valéri A, Rebillard X, Beuzeboc P, Richaud P, Soulié M, Salomon L (December 2009). "[Characteristics of prostate cancer in men less than 50-year-old]" (in French). Prog. Urol. 19 (11): 803–9. doi:10.1016/j.purol.2009.04.010. PMID 19945663.
- ↑ Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, Barry MJ, Zietman A, O'Leary M, Walker-Corkery E, Yao SL (September 2009). "Outcomes of Localized Prostate Cancer Following Conservative Management". The Journal of the American Medical Association 302 (11): 1202–09. doi:10.1001/jama.2009.1348. PMID 19755699.
- ↑ Mongiat-Artus P, Peyromaure M, Richaud P, Droz JP, Rainfray M, Jeandel C, Rebillard X, Moreau JL, Davin JL, Salomon L, Soulié M (December 2009). "[Recommendations for the treatment of prostate cancer in the elderly man: A study by the oncology committee of the French association of urology]" (in French). Prog. Urol. 19 (11): 810–7. doi:10.1016/j.purol.2009.02.008. PMID 19945664.
- ↑ Picard JC, Golshayan AR, Marshall DT, Opfermann KJ, Keane TE (November 2009). "The multi-disciplinary management of high-risk prostate cancer". Urol. Oncol.. doi:10.1016/j.urolonc.2009.09.002. PMID 19945310.
- ↑ Mouraviev V, Evans B, Polascik TJ (2006). "Salvage prostate cryoablation after primary interstitial brachytherapy failure: a feasible approach". Prostate Cancer Prostatic Dis. 9 (1): 99–101. doi:10.1038/sj.pcan.4500853. PMID 16314889.
- ↑ "Prostate Cancer At A Glance". ShaveMagazine.com. http://www.shavemagazine.com/body/health/090401/2.
- ↑ 48.0 48.1 [|Katz, Aaron] (2006). Guide to Prostate Health: From Conventional to Holistic Therapies. Freedom Press. ISBN 1-893910-37-7.
- ↑ Fan JK, Wei N, Ding M, Gu JF, Liu XR, Li BH, Qi R, Huang WD, Li YH, Xiong XQ, Wang J, Li RS, Liu XY (November 2009). "Targeting Gene-ViroTherapy for prostate cancer by DD3-driven oncolytic virus-harboring interleukin-24 gene". Int J Cancer 127 (3): NA. doi:10.1002/ijc.25069. PMID 19950222.
- ↑ Clarke (2005?). "Docetaxel for the Treatment of Hormone Refractory Prostate Cancer". http://www.nice.org.uk/nicemedia/live/11577/33321/33321.pdf.
- ↑ National Institute for Health and Clinical Excellence (NICE) Docetaxel for the treatment of hormone refractory prostate cancer
- ↑ "Avastin®, Thalomid®, Taxotere®, and Prednisone Effective for Men with Hormone Refractory Prostate Cancer". March 2010. http://professional.cancerconsultants.com/oncology_main_news.aspx?id=44815. Retrieved 10 May 2010.
- ↑ "Updated Survival Data on Provenge® for Treatment of Hormone Refractory Prostate Cancer Presented". http://professional.cancerconsultants.com/oncology_main_news.aspx?id=44732. Retrieved 9 May 2010.
- ↑ "Cabazitaxel Effective for Hormone Refractory Prostate Cancer After Failure of Taxotere®". http://professional.cancerconsultants.com/oncology_main_news.aspx?id=44709.
- ↑ ACS :: What Are The Risk Factors for Prostate Cancer?
- ↑ Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005). "Cancer statistics, 2005". CA Cancer J Clin 55 (1): 10–30. doi:10.3322/canjclin.55.1.10. PMID 15661684.
- ↑ Wakai K (February 2005). "[Descriptive epidemiology of prostate cancer in Japan and Western countries]" (in Japanese). Nippon Rinsho 63 (2): 207–12. PMID 15714967.
- ↑ Jaubert de Beaujeu M, Chavrier Y (January 1976). "[Deformations of the anterior thoracic wall (author's transl)]" (in French). Ann Chir Thorac Cardiovasc 15 (1): 1–6. PMID 1259345.
- ↑ Hsing AW, Tsao L, Devesa SS (January 2000). "International trends and patterns of prostate cancer incidence and mortality". Int. J. Cancer 85 (1): 60–7. doi:10.1002/(SICI)1097-0215(20000101)85:1<60::AID-IJC11>3.0.CO;2-B. PMID 10585584.
- ↑ Osegbe DN (April 1997). "Prostate cancer in Nigerians: facts and nonfacts". J. Urol. 157 (4): 1340–3. doi:10.1016/S0022-5347(01)64966-8. PMID 9120935.
- ↑ Di Blasio CJ, Rhee AC, Cho D, Scardino PT, Kattan MW (October 2003). "Predicting clinical end points: treatment nomograms in prostate cancer". Semin. Oncol. 30 (5): 567–86. doi:10.1016/S0093-7754(03)00351-8. PMID 14571407.
- ↑ Huggins C, Steven RE, Hodges CV (1941). "Studies on prostatic cancer". Arch. Surg. 43: 209–223.
- ↑ Hellerstedt BA, Pienta KJ (2002). "The current state of hormonal therapy for prostate cancer". CA Cancer J Clin 52 (3): 154–79. doi:10.3322/canjclin.52.3.154. PMID 12018929.
- ↑ Feldman BJ, Feldman D (October 2001). "The development of androgen-independent prostate cancer". Nat. Rev. Cancer 1 (1): 34–45. doi:10.1038/35094009. PMID 11900250.
- ↑ Cooperberg MR, Pasta DJ, Elkin EP, et al. (June 2005). "The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy". J. Urol. 173 (6): 1938–42. doi:10.1097/01.ju.0000158155.33890.e7. PMID 15879786.
- ↑ Cooperberg MR, Freedland SJ, Pasta DJ, et al. (November 2006). "Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy". Cancer 107 (10): 2384–91. doi:10.1002/cncr.22262. PMID 17039503.
- ↑ May M, Knoll N, Siegsmund M, et al. (November 2007). "Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy. Results from a european multicenter survey of 1,296 patients". J. Urol. 178 (5): 1957–62; discussion 1962. doi:10.1016/j.juro.2007.07.043. PMID 17868719.
- ↑ Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, Partin AW (August 2008). "External validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment score". Urology 72 (2): 396–400. doi:10.1016/j.urology.2007.11.165. PMID 18372031.
- ↑ Cooperberg MR, Broering JM, Carroll PR (June 2009). "Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis". J. Natl. Cancer Inst. 101 (12): 878–87. doi:10.1093/jnci/djp122. PMID 19509351.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html. Retrieved Nov. 11, 2009.
- ↑ Overview: Prostate Cancer—What Causes Prostate Cancer? American Cancer Society (2 May 2006). Retrieved on 5 April 2007
- ↑ Prostate Cancer FAQs. State University of New York School of Medicine Department of Urology (31 August 2006). Retrieved on 5 April 2007
- ↑ Potosky AL, Miller BA, Albertsen PC, Kramer BS (February 1995). "The role of increasing detection in the rising incidence of prostate cancer". JAMA 273 (7): 548–52. doi:10.1001/jama.273.7.548. PMID 7530782.
- ↑ Lippman SM, Klein EA, Goodman PJ, et al. (January 2009). "Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT)". JAMA 301 (1): 39–51. doi:10.1001/jama.2008.864. PMID 19066370.
- ↑ 75.0 75.1 75.2 Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA, Thompson IM (August 2008). "Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach". Cancer Prev Res (Phila Pa) 1 (3): 174–81. doi:10.1158/1940-6207.CAPR-08-0092. PMID 19138953.
- ↑ Gine Kolata (June 15, 2008). "New Take on a Prostate Drug, and a New Debate". NY Times. http://www.nytimes.com/2008/06/15/health/15prostate.html?ei=5087&em=&en=813eaa4e10f57756&ex=1213675200&adxnnl=1&adxnnlx=1213503418-GD4DbGjYsDxqV/xuGWnE1A. Retrieved 15 June 2008.
- ↑ Hsing AW, Chokkalingam AP (2006). "Prostate cancer epidemiology". Frontiers in Bioscience 11: 1388–413. doi:10.2741/1891. PMID 16368524.
- ↑ Hankey, BF; Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Kaplan RS (June 16 1999). "Cancer surveillance series: interpreting trends in prostate cancer—part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates". J Natl Cancer Inst 91 (12): 1017–24. doi:10.1093/jnci/91.12.1017. PMID 10379964.
- ↑ Breslow, N; Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, Tulinius H. (November 15 1977). "Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France". Int J Cancer 20 (5): 680–8. doi:10.1002/ijc.2910200506. PMID 924691.
- ↑ Zeegers MP (2003). "Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis". Cancer 97: 1894-903. PMID 12673715.
- ↑ Jemal A, A; Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (Jan-February 2005). "Cancer statistics, 2005". CA Cancer J Clin 55 (1): 10–30. doi:10.3322/canjclin.55.1.10. PMID 15661684. Erratum in: CA Cancer J Clin. 2005 Jul-Aug;55(4):259
- ↑ Martin RM, Vatten L, Gunnell D, Romundstad P (March 2010). "Blood pressure and risk of prostate cancer: cohort Norway (CONOR)". Cancer Causes Control 21 (3): 463–72. doi:10.1007/s10552-009-9477-x. PMID 19949849.
- ↑ Witte ON, et al. (July 2010). "Growth Regulation of Hematopoietic and Epithelial Cancers and the Immune Response". Science 329 (5991): 568-571. http://www.sciencemag.org/cgi/content/abstract/sci;329/5991/568?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=prostate+cancer&searchid=1&FIRSTINDEX=.
- ↑ 84.0 84.1 Gallagher RP, Fleshner N (October 1998). "Prostate cancer: 3. Individual risk factors". CMAJ 159 (7): 807–13. PMID 9805030. PMC 1232741. http://www.cmaj.ca/cgi/reprint/159/7/807.pdf.
- ↑ Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL (March 2001). "Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study". J. Natl. Cancer Inst. 93 (5): 388–95. doi:10.1093/jnci/93.5.388. PMID 11238701.
- ↑ Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC (1990). "Family history and the risk of prostate cancer". Prostate 17 (4): 337–47. doi:10.1002/pros.2990170409. PMID 2251225.
- ↑ Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K (July 2000). "Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland". N. Engl. J. Med. 343 (2): 78–85. doi:10.1056/NEJM200007133430201. PMID 10891514.
- ↑ Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA (May 1997). "The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews". N. Engl. J. Med. 336 (20): 1401–8. doi:10.1056/NEJM199705153362001. PMID 9145676.
- ↑ Beuzeboc P, Soulié M, Richaud P, Salomon L, Staerman F, Peyromaure M, Mongiat-Artus P, Cornud F, Paparel P, Davin JL, Molinié V (December 2009). "[Fusion genes and prostate cancer. From discovery to prognosis and therapeutic perspectives]" (in French). Prog. Urol. 19 (11): 819–24. doi:10.1016/j.purol.2009.06.002. PMID 19945666.
- ↑ "Scientists Discover Anti-Cancer Mechanism that Arrests Early Prostate Cancer". August 4, 2005. http://www.mskcc.org/mskcc/html/59384.cfm.
- ↑ Kasperzyk JL, Fall K, Mucci LA, et al. (September 2009). "One-carbon metabolism-related nutrients and prostate cancer survival". Am. J. Clin. Nutr. 90 (3): 561–9. doi:10.3945/ajcn.2009.27645. PMID 19571228. PMC 2728642. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=19571228.
- ↑ Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, Gelmann EP, Friesen MD, Riboli E, Hayes RB (2007). "Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial". Cancer Epidemiol. Biomarkers Prev. 16 (5): 962–8. doi:10.1158/1055-9965.EPI-06-0861. PMID 17507623. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=17507623.
- ↑ Wigle DT, Turner MC, Gomes J, Parent ME (March 2008). "Role of hormonal and other factors in human prostate cancer". Journal of Toxicology and Environmental Health. Part B, Critical Reviews 11 (3-4): 242–59. doi:10.1080/10937400701873548. PMID 18368555.
- ↑ Schulman, CC; Ekane S; Zlotta AR (September 2001). "Nutrition and prostate cancer: evidence or suspicion?". Urology 58 (3): 318–34. doi:10.1016/S0090-4295(01)01262-6. PMID 11549473.
- ↑ Brinkman M, Reulen RC, Kellen E, Buntinx F, & Zeegers MP (2006). "Are men with low selenium levels at increased risk of prostate cancer?". European Journal of Cancer 42 (15): 2463–2471. doi:10.1016/j.ejca.2006.02.027. PMID 16945521.
- ↑ Duffield-Lillico AJ, et al. (2002). "Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial". Cancer Epidemiology, Biomarkers, and Prevention 11: 630–693.
- ↑ Brinkman M, Zeegers MP (2006). "Are men with low selenium levels at increased risk of prostate cancer?". Eur J Cancer 42 (15): 2463-71. PMID 16945521.
- ↑ Lippman SM, et al. (2008). "Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT)". Journal of American Medical Association 301 (1): 39–51. doi:10.1001/jama.2008.864. PMID 19066370.
- ↑ Klein EA, et al. (2001). "SELECT: The next prostate cancer prevention trial-Selenium and Vitamin E Cancer Prevention Trial.". Journal of Urology 166 (4): 1311–1315. PMID 11547064.
- ↑ 100.0 100.1 Portes-Antoine S., & France de Bravo B. (August 2009). "Prostate Cancer: Diet and Dietary Supplements". Cancer Prevention and Treatment Fund. http://www.stopcancerfund.org/posts/207. Retrieved January 12, 2010.
- ↑ Chan JM, Oh WK, Xie W, Regan MM, Stampfer MJ, King IB, Abe M, Kantoff PW (August 2009). "Plasma selenium, manganese superoxide dismutase, and intermediate- or high-risk prostate cancer". J. Clin. Oncol. 27 (22): 3577–83. doi:10.1200/JCO.2008.18.8938. PMID 19528373.
- ↑ Lee AH, Fraser ML, Meng X, Binns CW (April 2006). "Protective effects of green tea against prostate cancer". Expert Rev Anticancer Ther 6 (4): 507–13. doi:10.1586/14737140.6.4.507. PMID 16613539.
- ↑ Kikuchi N, Ohmori K, Shimazu T, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y, Tsuji I (August 2006). "No association between green tea and prostate cancer risk in Japanese men: the Ohsaki Cohort Study". Br. J. Cancer 95 (3): 371–3. doi:10.1038/sj.bjc.6603230. PMID 16804523.
- ↑ "Multivitamin prostate warning". Health. BBC NEWS. 16 May 2007. http://news.bbc.co.uk/1/hi/health/6657795.stm.
- ↑ Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, Leitzmann MF (May 2007). "Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study". J. Natl. Cancer Inst. 99 (10): 754–64. doi:10.1093/jnci/djk177. PMID 17505071.
- ↑ "Broccoli May Help Cut Prostate Cancer, Broccoli, Cauliflower May Make Aggressive Prostate Cancer Less Likely". CBS News. 24 July 2007. http://www.cbsnews.com/stories/2007/07/24/health/webmd/main3094509.shtml.
- ↑ Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB (August 2007). "Prospective study of fruit and vegetable intake and risk of prostate cancer". J. Natl. Cancer Inst. 99 (15): 1200–9. doi:10.1093/jnci/djm065. PMID 17652276.
- ↑ Sarkar FH, Li Y (December 2004). "Indole-3-carbinol and prostate cancer". J. Nutr. 134 (12 Suppl): 3493S–8S. PMID 15570059.
- ↑ Hsu JC, Zhang J, Dev A, Wing A, Bjeldanes LF, Firestone GL (November 2005). "Indole-3-carbinol inhibition of androgen receptor expression and downregulation of androgen responsiveness in human prostate cancer cells". Carcinogenesis 26 (11): 1896–904. doi:10.1093/carcin/bgi155. PMID 15958518.
- ↑ 110.0 110.1 110.2 110.3 Poon KS, McVary KT. Curr Urol Rep. 2009 Jul;10(4):279-86. Dietary patterns, supplement use, and the risk of benign prostatic hyperplasia.
- ↑ 111.0 111.1 Hultdin J, Van Guelpen B, Bergh A, Hallmans G, Stattin P (February 2005). "Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study". International Journal of Cancer 113 (5): 819–24. doi:10.1002/ijc.20646. PMID 15499634.
- ↑ 112.0 112.1 112.2 112.3 112.4 112.5 Shannon J, Phoutrides E, Palma A, Farris P, Peters L, Forester A, Tillotson CJ, Garzotto M (2009). "Folate intake and prostate cancer risk: a case-control study". Nutr Cancer 61 (5): 617–28. doi:10.1080/01635580902846593. PMID 19838935.
- ↑ 113.0 113.1 Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL (July 2003). "Zinc supplement use and risk of prostate cancer". J. Natl. Cancer Inst. 95 (13): 1004–7. doi:10.1093/jnci/95.13.1004. PMID 12837837.
- ↑ Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ (July 2005). "A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence". J. Natl. Cancer Inst. 97 (13): 975–80. doi:10.1093/jnci/dji173. PMID 15998950.
- ↑ Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE (August 2005). "Statins and prostate cancer risk: a case-control study". Am. J. Epidemiol. 162 (4): 318–25. doi:10.1093/aje/kwi203. PMID 16014776.
- ↑ Dennis LK, Lynch CF, Torner JC (July 2002). "Epidemiologic association between prostatitis and prostate cancer". Urology 60 (1): 78–83. doi:10.1016/S0090-4295(02)01637-0. PMID 12100928.
- ↑ Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (April 2003). "Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults". N. Engl. J. Med. 348 (17): 1625–38. doi:10.1056/NEJMoa021423. PMID 12711737.
- ↑ Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ (August 1996). "Prospective study of sex hormone levels and risk of prostate cancer". J. Natl. Cancer Inst. 88 (16): 1118–26. doi:10.1093/jnci/88.16.1118. PMID 8757191.
- ↑ "?". http://www.bccancer.bc.ca. Retrieved 9 August 2010.
- ↑ "Veterans exposed to Agent Orange have higher rates of prostate cancer recurrence". Medical College of Georgia News. May 20, 2007. https://my.mcg.edu/portal/page/portal/News/archive/2007/Veterans%20exposed%20to%20Agent%20%20Orange%20have%20higher%20rates%20of%20prost.
- ↑ Urisman A, Molinaro RJ, Fischer N, Plummer SJ, Casey G, Klein EA, Malathi K, Magi-Galluzzi C, Tubbs RR, Ganem D, Silverman RH, DeRisi JL (March 2006). "Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant". PLoS Pathog. 2 (3): e25. doi:10.1371/journal.ppat.0020025. PMID 16609730.
- ↑ Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR (September 2009). "XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors". Proc. Natl. Acad. Sci. U.S.A. 106 (38): 16351–6. doi:10.1073/pnas.0906922106. PMID 19805305.
- ↑ Hohn O, Krause H, Barbarotto P, Niederstadt L, Beimforde N, Denner J, Miller K, Kurth R, Bannert N (2009). "Lack of evidence for xenotropic murine leukemia virus-related virus(XMRV) in German prostate cancer patients". Retrovirology 6: 92. doi:10.1186/1742-4690-6-92. PMID 19835577.
- ↑ Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA (July 2003). "The influence of finasteride on the development of prostate cancer". N. Engl. J. Med. 349 (3): 215–24. doi:10.1056/NEJMoa030660. PMID 12824459.
- ↑ Andriole GL, Roehrborn C, Schulman C, Slawin KM, Somerville M, Rittmaster RS (September 2004). "Effect of dutasteride on the detection of prostate cancer in men with benign prostatic hyperplasia". Urology 64 (3): 537–41; discussion 542–3. doi:10.1016/j.urology.2004.04.084. PMID 15351586.
- ↑ Giles GG, Severi G, English DR, McCredie MR, Borland R, Boyle P, Hopper JL (August 2003). "Sexual factors and prostate cancer". BJU Int. 92 (3): 211–6. doi:10.1046/j.1464-410X.2003.04319.x. PMID 12887469.
- ↑ Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E (April 2004). "Ejaculation frequency and subsequent risk of prostate cancer". JAMA 291 (13): 1578–86. doi:10.1001/jama.291.13.1578. PMID 15069045.
- ↑ Douglas Fox (16 July 2003). "Masturbating may protect against prostate cancer". New Scientist. http://www.newscientist.com/article/dn3942-masturbating-may-protect-against-prostate-cancer.html.
- ↑ Dimitropoulou P, Lophatananon A, Easton D, Pocock R, Dearnaley DP, Guy M, Edwards S, O'Brien L, Hall A, Wilkinson R, Eeles R, Muir KR (January 2009). "Sexual activity and prostate cancer risk in men diagnosed at a younger age". BJU Int. 103 (2): 178–85. doi:10.1111/j.1464-410X.2008.08030.x. PMID 19016689.
- ↑ Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D'Agostino R, Zhang H, Wu H, Kang JX, Chen YQ (July 2007). "Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids". J. Clin. Invest. 117 (7): 1866–75. doi:10.1172/JCI31494. PMID 17607361.
- ↑ 131.0 131.1 131.2 Männistö S, Pietinen P, Virtanen MJ, et al. (December 2003). "Fatty acids and risk of prostate cancer in a nested case-control study in male smokers". Cancer Epidemiology, Biomarkers & Prevention 12 (12): 1422–8. PMID 14693732. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=14693732.
- ↑ Gann, PH and Giovannucci (2005). "Prostate Cancer and Nutrition" (PDF). http://www.prostatecancerfoundation.org/atf/cf/%7B705B3273-F2EF-4EF6-A653-E15C5D8BB6B1%7D/Nutrition_Guide.pdf. Retrieved February 20, 2006. in .pdf format.
- ↑ Chavarro JE, Stampfer MJ, Campos H, Kurth T, Willett WC, Ma J (January 2008). "A prospective study of trans-fatty acid levels in blood and risk of prostate cancer". Cancer Epidemiol. Biomarkers Prev. 17 (1): 95–101. doi:10.1158/1055-9965.EPI-07-0673. PMID 18199715.
- ↑ 134.0 134.1 134.2 Crowe FL, Allen NE, Appleby PN, et al. (November 2008). "Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition". The American Journal of Clinical Nutrition 88 (5): 1353–63. PMID 18996872. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=18996872.
- ↑ 135.0 135.1 Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS (April 2008). "Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men". Cancer Epidemiology, Biomarkers & Prevention 17 (4): 930–7. doi:10.1158/1055-9965.EPI-07-2681. PMID 18398033.
- ↑ American Dietetic Association and Dieticians of Canada (June 2003). "Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets". Journal of the American Dietetic Association 103 (6): 748–65. doi:10.1053/jada.2003.50142. PMID 12778049.
- ↑ Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, Cohen P, Hwang D, Peterson B, Fields T, Pizzo SV, Isaacs WB (January 2008). "Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis". Prostate 68 (1): 11–9. doi:10.1002/pros.20683. PMID 17999389.
- ↑ "Can Coffee Lower The Risk of Prostate Cancer?". http://www.npr.org/templates/story/story.php?storyId=121343438.
- ↑ Adams J (1853). "The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis". Lancet 1.
- ↑ Lytton B (June 2001). "Prostate cancer: a brief history and the discovery of hormonal ablation treatment". The Journal of Urology 165 (6 Pt 1): 1859–62. doi:10.1016/S0022-5347(05)66228-3. PMID 11371867.
- ↑ Young HH (1905). "Four cases of radical prostatectomy". Johns Hopkins Bull. 16: 315.
- ↑ Walsh PC, Lepor H, Eggleston JC (1983). "Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations". The Prostate 4 (5): 473–85. doi:10.1002/pros.2990040506. PMID 6889192.
- ↑ Huggins CB, Hodges CV (1941). "Studies on prostate cancer: 1. The effects of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate". Cancer Res 1.
- ↑ Schally AV, Kastin AJ, Arimura A (November 1971). "Hypothalamic follicle-stimulating hormone (FSH) and luteinizing hormone (LH)-regulating hormone: structure, physiology, and clinical studies". Fertility and Sterility 22 (11): 703–21. PMID 4941683.
- ↑ Tolis G, Ackman D, Stellos A, et al. (March 1982). "Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists". Proc. Natl. Acad. Sci. U.S.A 79 (5): 1658–62. doi:10.1073/pnas.79.5.1658. PMID 6461861.
- ↑ Denmeade SR, Isaacs JT (May 2002). "A history of prostate cancer treatment". Nature Reviews. Cancer 2 (5): 389–96. doi:10.1038/nrc801. PMID 12044015.
- ↑ Scott WW, Johnson DE, Schmidt JE, et al. (December 1975). "Chemotherapy of advanced prostatic carcinoma with cyclophosphamide or 5-fluorouracil: results of first national randomized study". The Journal of Urology 114 (6): 909–11. PMID 1104900.
- ↑ Witte ON, et al. (July 2010). "Growth Regulation of Hematopoietic and Epithelial Cancers and the Immune Response". Science 329 (5991): 568-571.
- ↑ Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S (March 2005). "Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice". Cancer Res. 65 (6): 2082–4. doi:10.1158/0008-5472.CAN-04-3992. PMID 15781616.
- ↑ Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S (July 2006). "Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist". Cancer Res. 66 (13): 6482–6. doi:10.1158/0008-5472.CAN-06-0632. PMID 16818617.
- ↑ Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H (August 2001). "Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols". Proc. Natl. Acad. Sci. U.S.A. 98 (18): 10350–5. doi:10.1073/pnas.171326098. PMID 11504910.
- ↑ Grotendorst GR, Lau LF, Perbal B (June 2000). "CCN proteins are distinct from and should not be considered members of the insulin-like growth factor-binding protein superfamily". Endocrinology 141 (6): 2254–6. doi:10.1210/en.141.6.2254. PMID 10830315.
- ↑ Samani AA, Yakar S, LeRoith D, Brodt P (February 2007). "The role of the IGF system in cancer growth and metastasis: overview and recent insights". Endocrine Reviews 28 (1): 20–47. doi:10.1210/er.2006-0001. PMID 16931767.
- ↑ Jenkins PJ, Bustin SA (August 2004). "Evidence for a link between IGF-I and cancer". European Journal of Endocrinology 151 (Suppl 1): S17–22. doi:10.1530/eje.0.151S017. PMID 15339239.
- ↑ Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T (May 2001). "Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer". Cancer Research 61 (9): 3550–5. PMID 11325816. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=11325816.
- ↑ Ford OH, Gregory CW, Kim D, Smitherman AB, Mohler JL (November 2003). "Androgen receptor gene amplification and protein expression in recurrent prostate cancer". The Journal of Urology 170 (5): 1817–21. doi:10.1097/01.ju.0000091873.09677.f4. PMID 14532783.
- ↑ Kokontis J, Takakura K, Hay N, Liao S (March 1994). "Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation". Cancer Research 54 (6): 1566–73. PMID 7511045. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=7511045.
- ↑ Umekita Y, Hiipakka RA, Kokontis JM, Liao S (October 1996). "Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride". Proc. Natl. Acad. Sci. U.S.A 93 (21): 11802–7. doi:10.1073/pnas.93.21.11802. PMID 8876218.
- ↑ Kokontis JM, Hsu S, Chuu CP, et al. (December 2005). "Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity". The Prostate 65 (4): 287–98. doi:10.1002/pros.20285. PMID 16015608.
External links
- Prostate Cancer Clinical Trials Search and learn about clinical trials for prostate cancer
- Prostate Cancer at American Cancer Society
- Prostate Cancer From Cancer Management: A Multidisciplinary Approach
- Prostate Cancer & Endothelin: PMAP The Proteolysis Map-animation
- Prostate cancer at the Open Directory Project
- Interactive Health Tutorials Medline Plus: What's Prostate Cancer Using animated graphics and you can also listen to the tutorial
- Highlights of ASCO 2007 Prostate Symposium, Anna Ferrari. Overview for doctors of ongoing European and U.S. clinical studies of prostate cancer presented at the New York Cancer Consortium. Data on screening, and treatment of local, intermediate and advanced prostate cancer by surgery, radiation and hormones. Answering the question, "What do we tell our patients about treatment decisions?"
- [2] "Prostate Cancer Treatments"
- Prostate Cancer Prevention - World Foundation of Urology
|
||||||||||||||||||||||||||||||||||